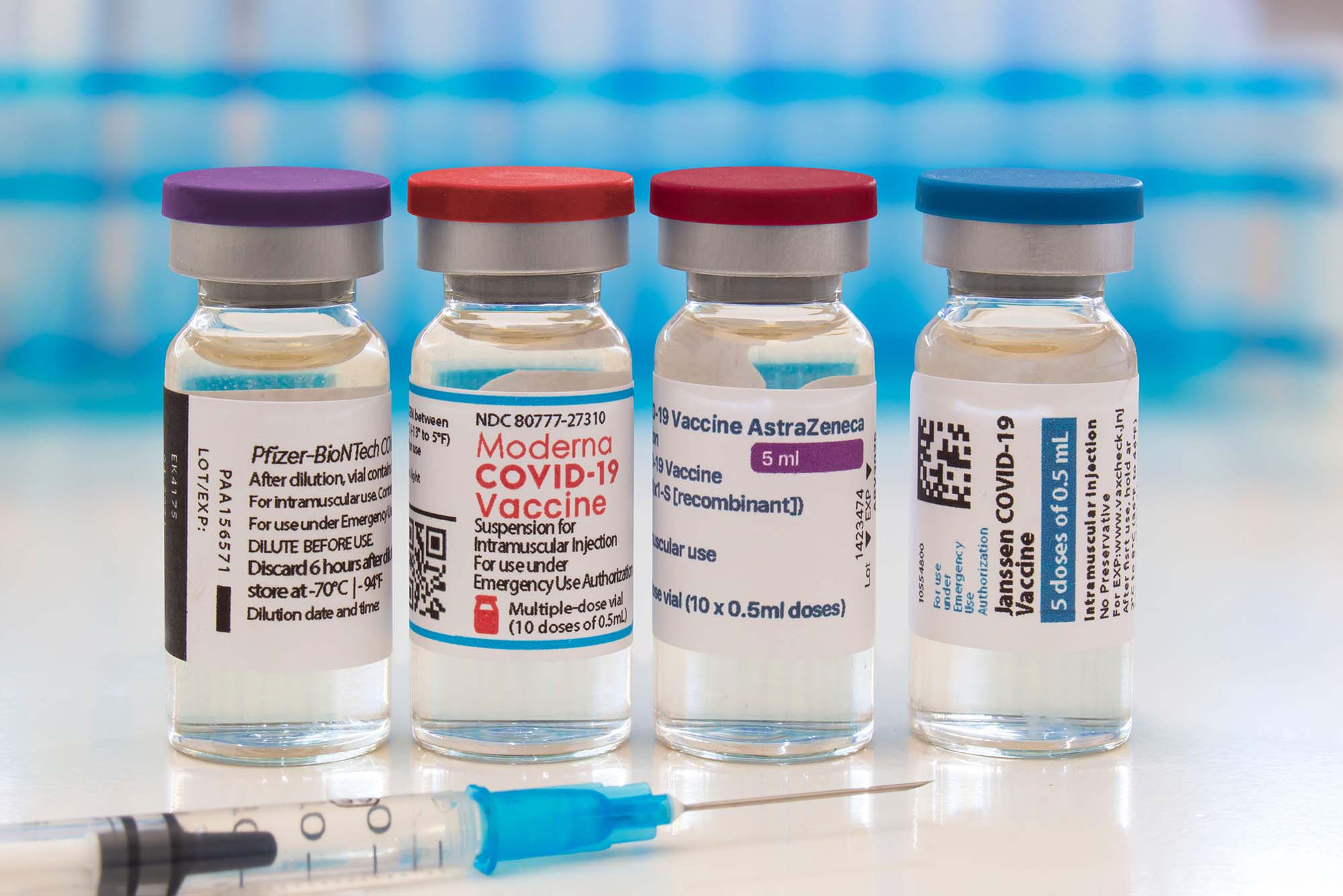
FDA authorizes another booster dose of the Pfizer or Moderna COVID-19 vaccine for people age 50 and up - The Boston Globe
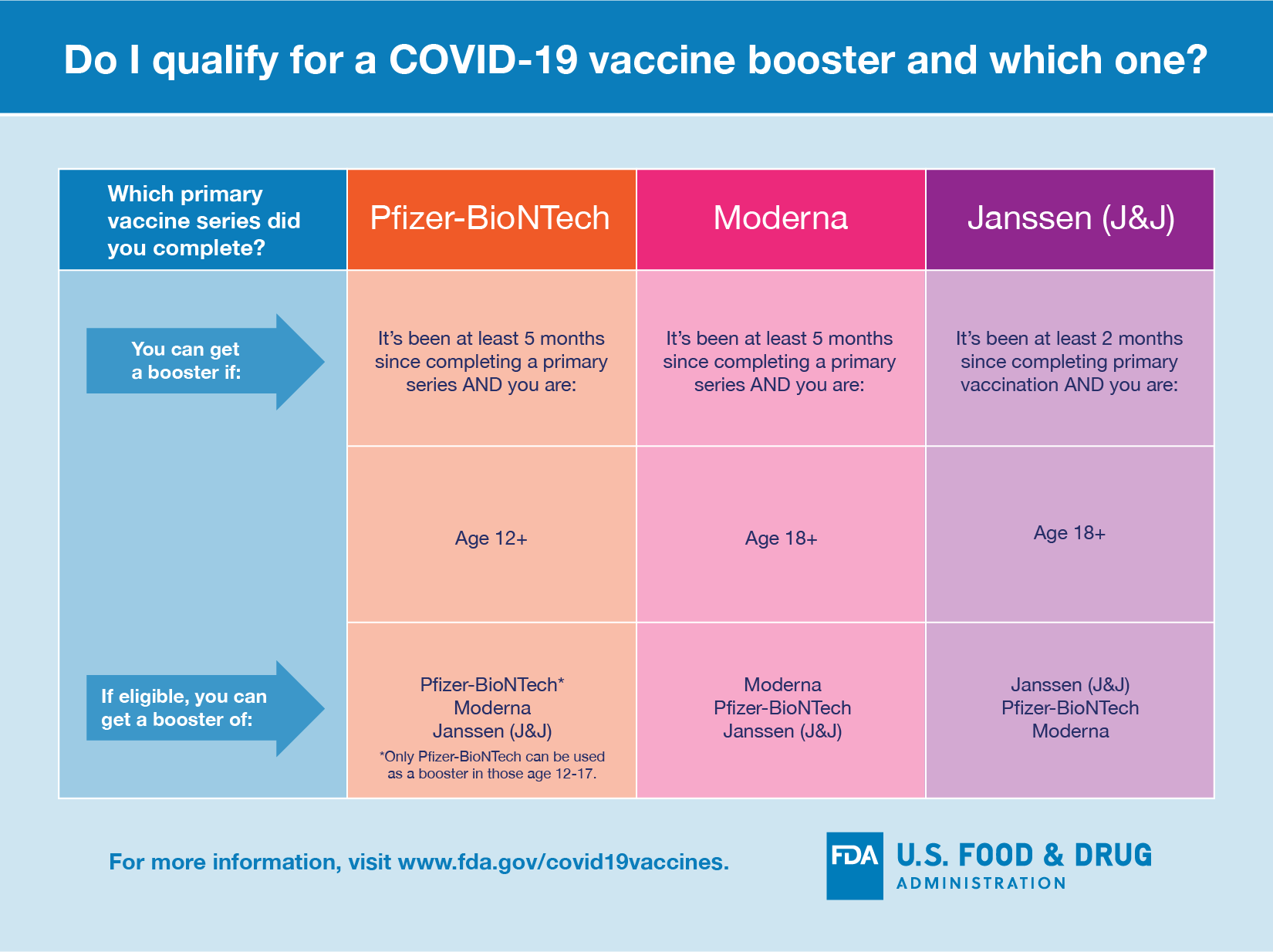
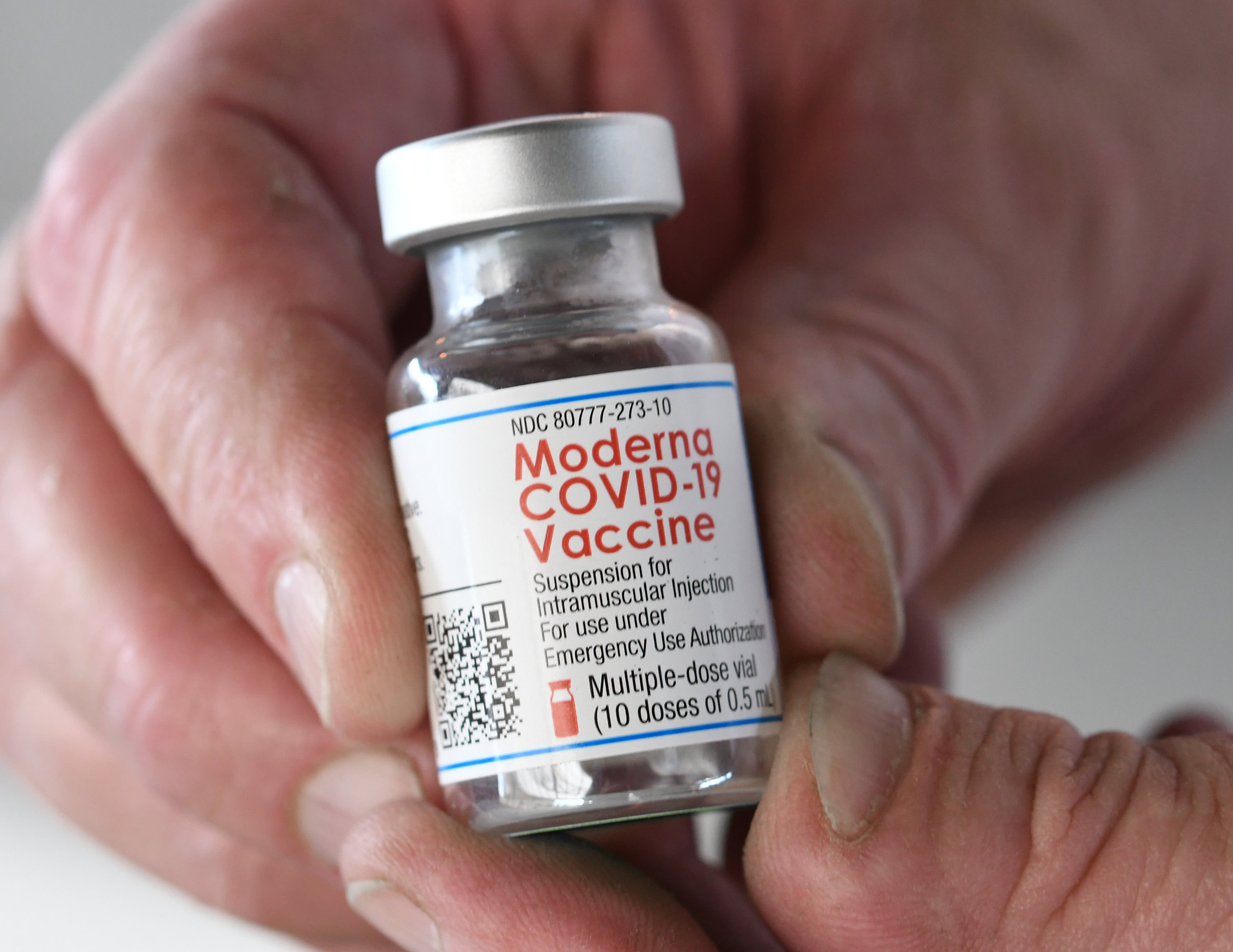
Coronavirus (COVID-19) Update: FDA Shortens Interval for Booster Dose of Moderna COVID-19 Vaccine to Five Months | FDA
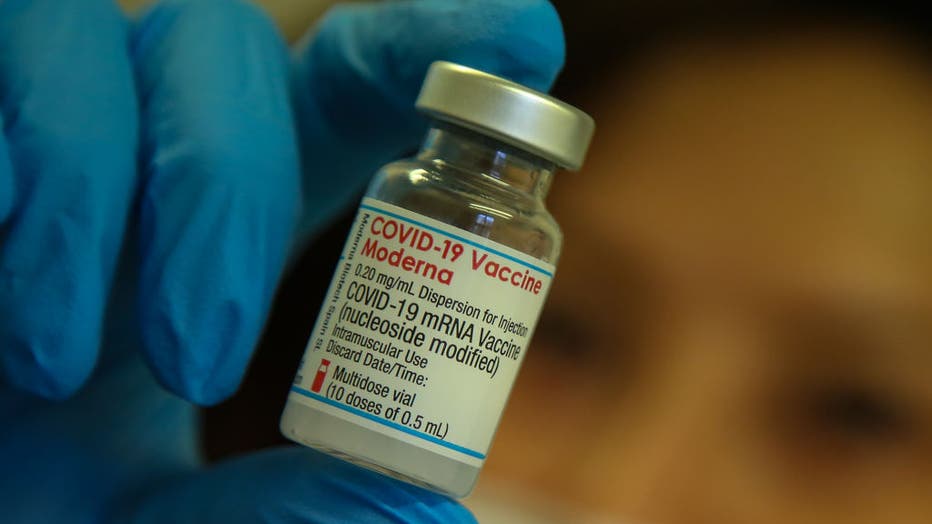
OCHD ANNOUNCES MODERNA AND J&J BOOSTER AVAILABLE AT VACCINATION CLINICS FOR ELIGIBLE RESIDENTS – Ocean County Health Department
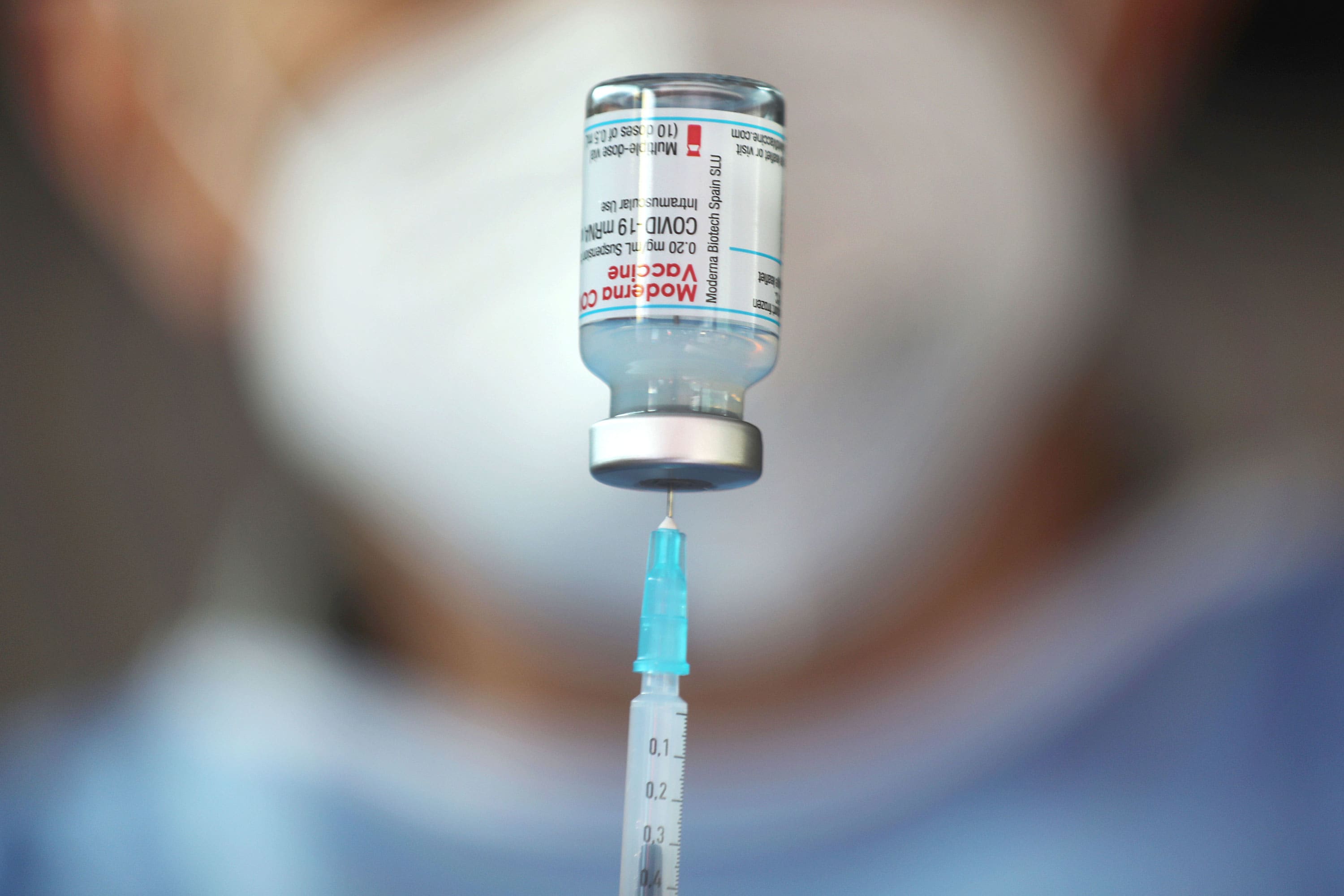
CDC recommends new COVID booster for all Americans over 6 months amid rising cases, hospitalizations - ABC News