U.S. FDA on X: "Today, we amended the emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 Vaccine to allow for use of a single booster dose, to be administered at least 6
FDA weighs booster shot recommendations as COVID-19 cases see slight dip – Zilber College of Public Health
FDA Advisers Say Next Round of COVID Booster Shots Should Target an XBB Variant - Southern Iowa Mental Health Center
Pfizer asks FDA to authorize Covid booster shots that target omicron BA.5 for people ages 12 and older

FDA committee meets to debate and vote on Covid booster shots for the general public — 9/17/21 - YouTube

FDA panel rejects broad use of COVID-19 boosters, approves extra doses for seniors, those at high risk | CBC News

CDC recommends new COVID booster for all Americans over 6 months amid rising cases, hospitalizations - ABC News
Tense decision-making as CDC joins FDA in recommending Pfizer booster shot for 65 & up, people at high risk and those with occupational exposure to COVID-19
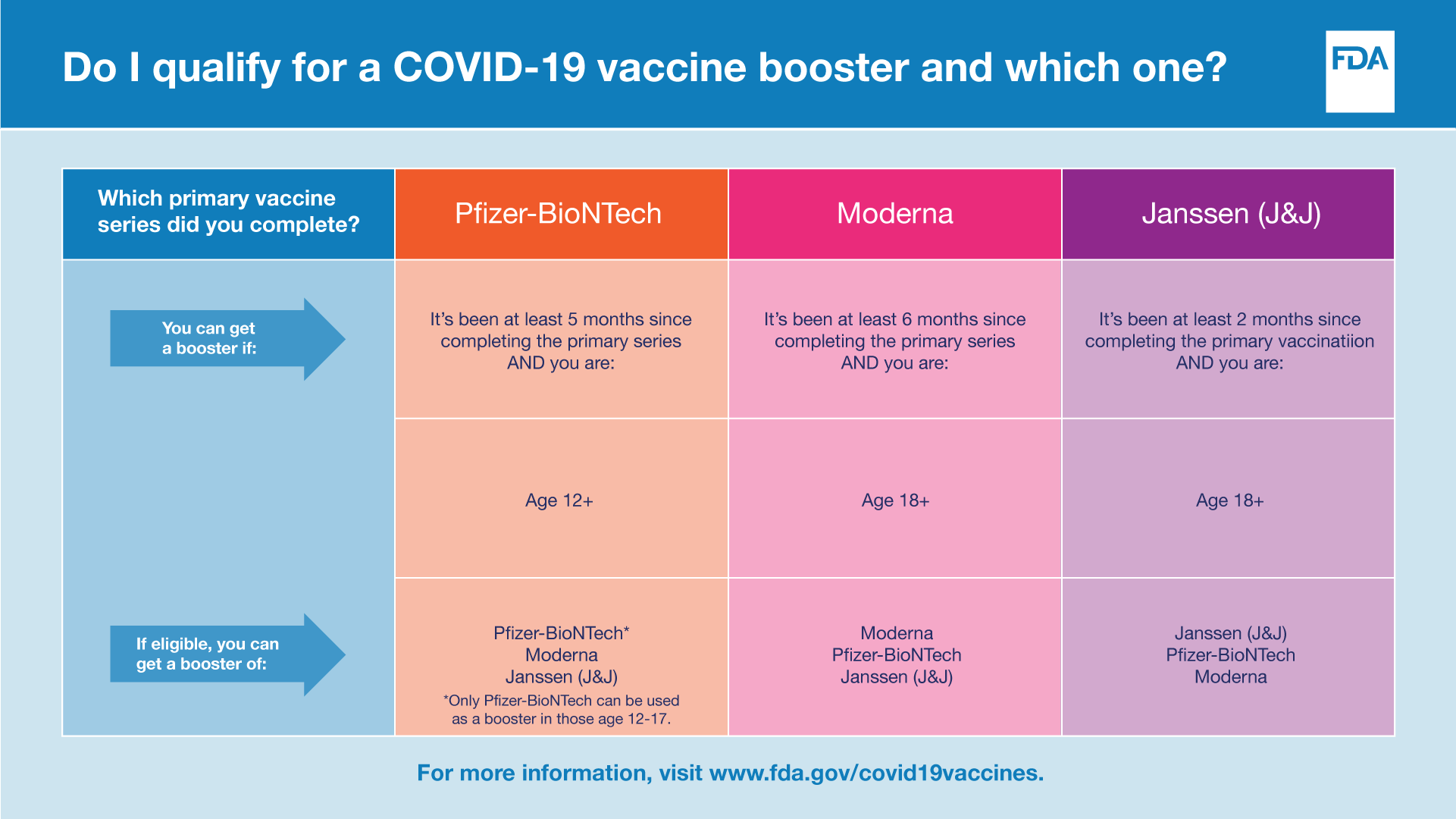
Coronavirus (COVID-19) Update: FDA Takes Multiple Actions to Expand Use of Pfizer-BioNTech COVID-19 Vaccine | FDA
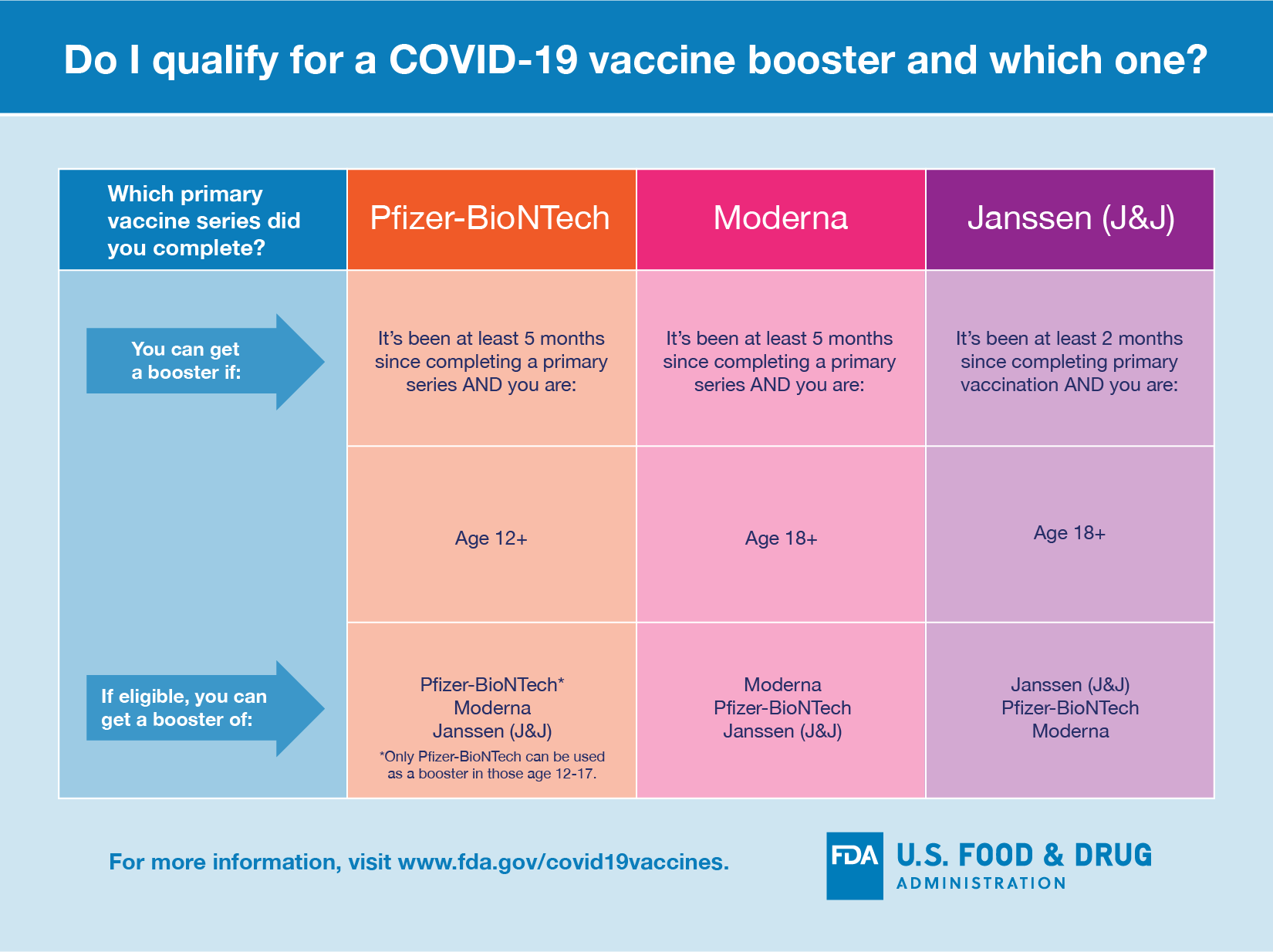
Coronavirus (COVID-19) Update: FDA Shortens Interval for Booster Dose of Moderna COVID-19 Vaccine to Five Months | FDA