Novavax: risultati positivi per vaccino Covid come booster e per vaccino combinato Covid-Influenza – Daily Health Industry
FIMMG Bari - Arriva il Novavax; solo per il ciclo primario agli over 18 (2 dosi) e non per i booster
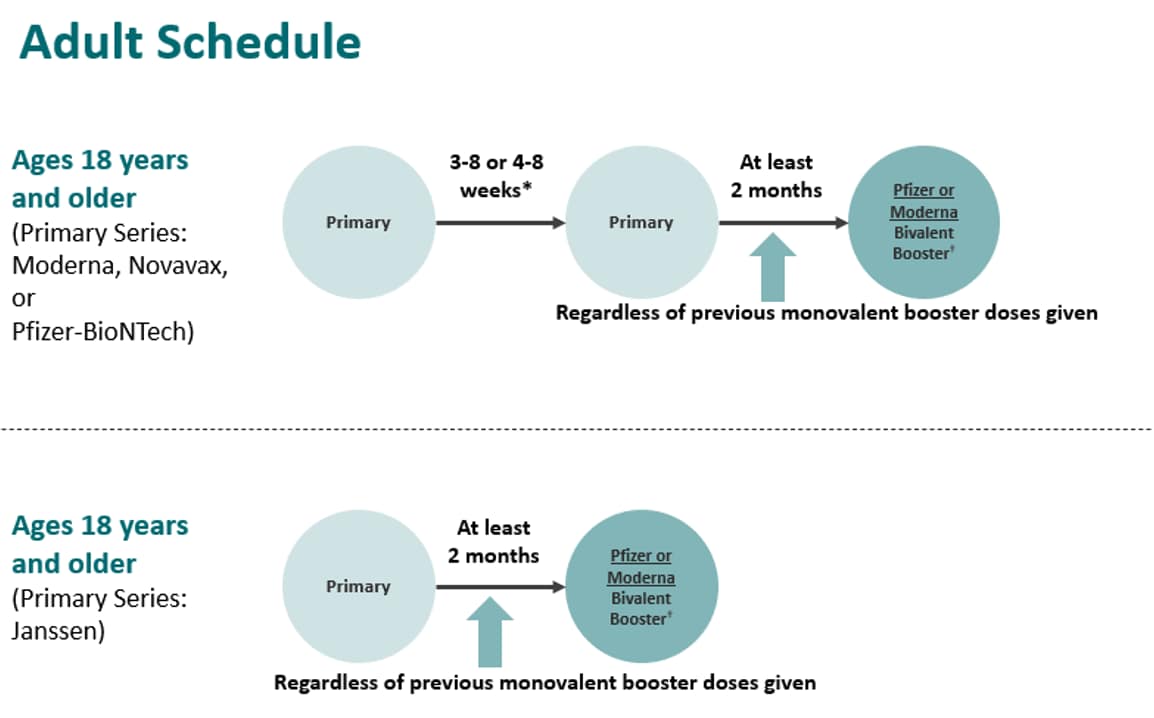
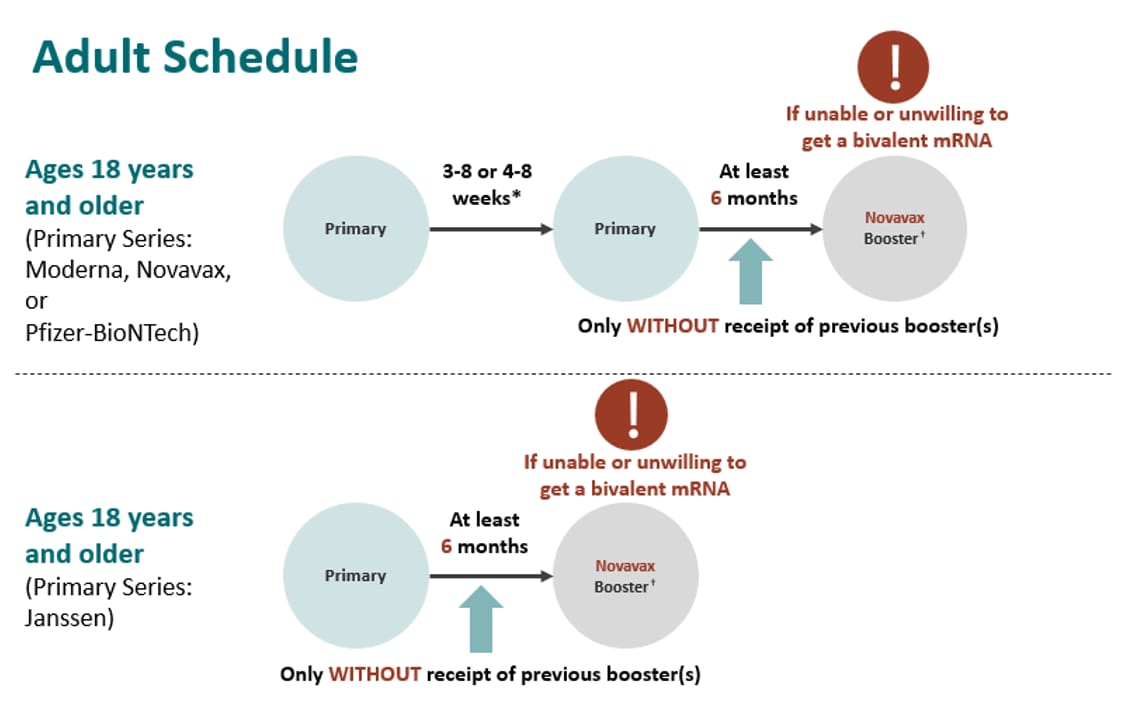
ACIP Evidence to Recommendations (EtR) for Use of Novavax COVID-19 Vaccine Booster Dose for adults ages 18 years and older under an Emergency Use Authorization | CDC
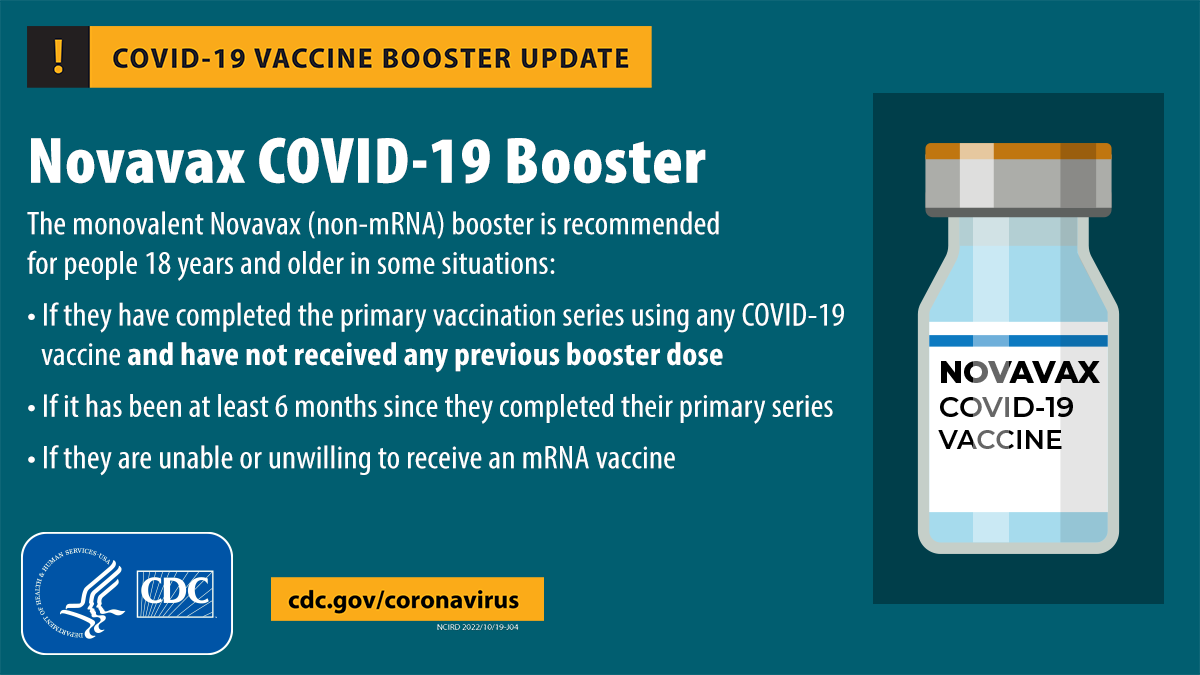
CDC on X: "CDC recommends Novavax's non-mRNA booster for people ages 18+, for certain situations. This includes those unable or unwilling to receive mRNA #COVID19 vaccines. The Novavax COVID-19 booster targets the