Department of Health and Social Care on Twitter: "Everyone aged 16 and over is eligible for the #COVID19 booster from three months after their second dose. Book an appointment online or find

Immunogenicity and safety of NVSI-06-07 as a heterologous booster after priming with BBIBP-CorV: a phase 2 trial | Signal Transduction and Targeted Therapy

Cohort Profile:The Danish National Cohort Study of Effectiveness and Safety of SARS-CoV-2 vaccines (ENFORCE) | BMJ Open

Safety and immunogenicity of seven COVID-19 vaccines as a third dose ( booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV- BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial -

Safety and immunogenicity of a bivalent SARS-CoV-2 protein booster vaccine, SCTV01C, in adults previously vaccinated with mRNA vaccine: a randomized, double-blind, placebo-controlled phase 1/2 clinical trial - eBioMedicine
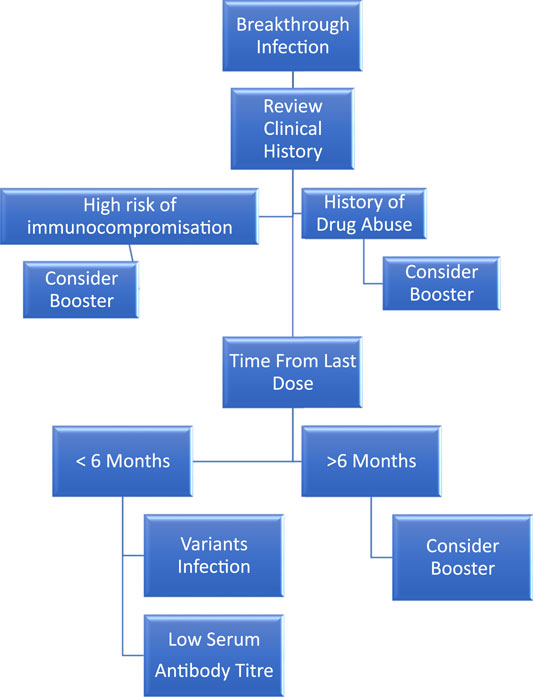
Frontiers | Review of Clinical Trials of COVID-19 Vaccination Booster in SARS-CoV-2 Variants Era: To Take It or Not To Take It

Pneumococcal conjugate vaccine 13 delivered as one primary and one booster dose (1 + 1) compared with two primary doses and a booster (2 + 1) in UK infants: a multicentre, parallel
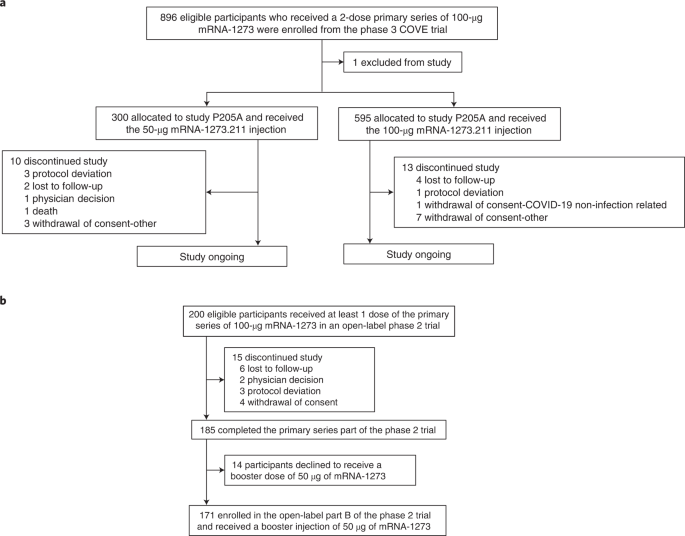
Safety, immunogenicity and antibody persistence of a bivalent Beta-containing booster vaccine against COVID-19: a phase 2/3 trial | Nature Medicine

Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial - The Lancet

Immunogenicity of a single-dose compared with a two-dose primary series followed by a booster dose of ten-valent or 13-valent pneumococcal conjugate vaccine in South African children: an open-label, randomised, non-inferiority trial -
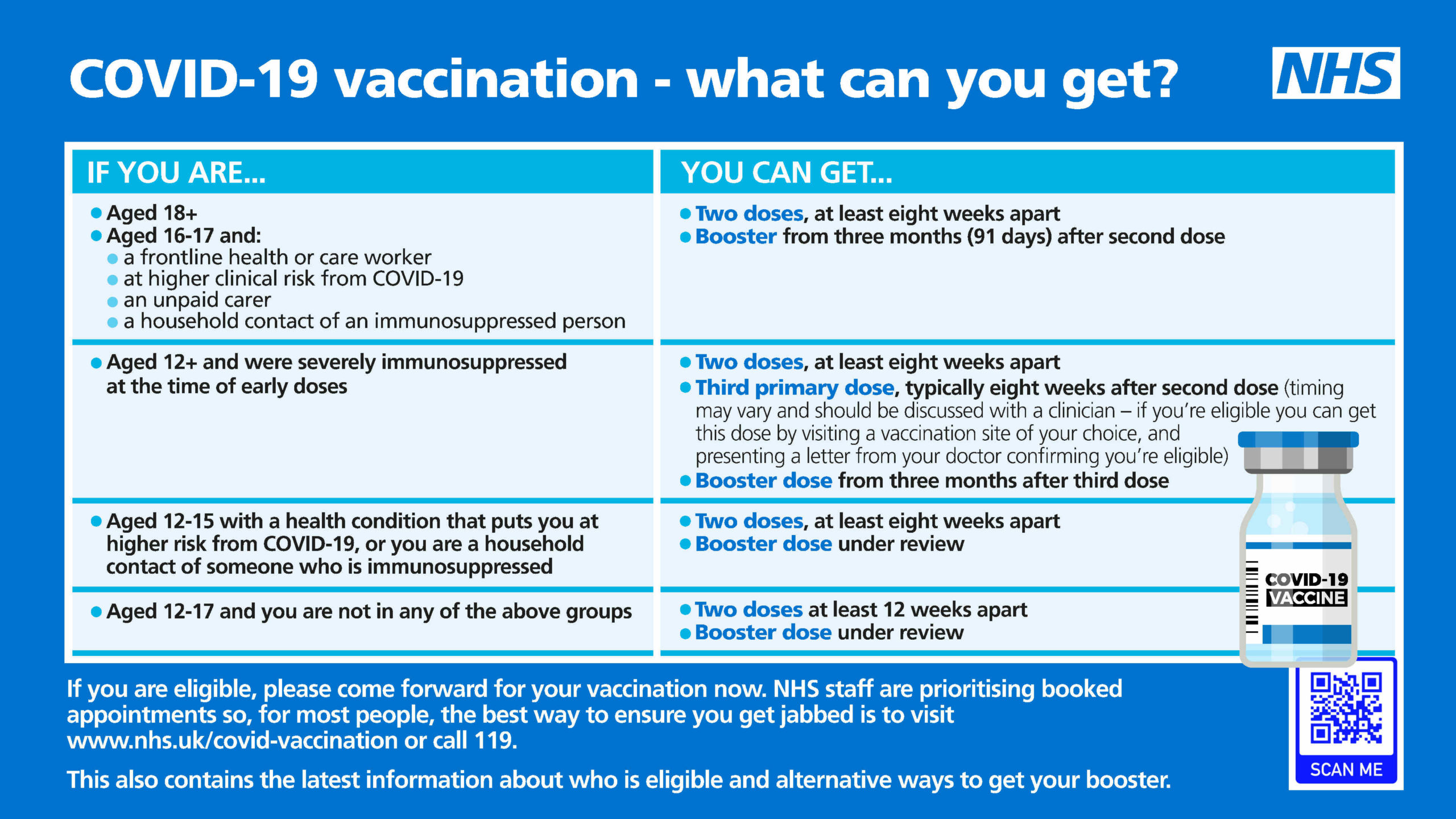
COVID vaccinations: questions and answers about the rollout in North Yorkshire - NHS North Yorkshire CCG