News - CHMP Recommends Additional Authorisation Modification of Comirnaty (BioNTech/Pfizer) As a Bivalent Vaccine Adapted to Omicron BA.4/BA.5 for Booster Vaccinations - Paul-Ehrlich-Institut
Common Questions About Bivalent COVID-19 Boosters | Johns Hopkins | Bloomberg School of Public Health
News - CHMP Recommends Authorisation of Comirnaty Variant (BioNTech/Pfizer) Adapted to Omicron BA.4/BA.5 As Booster Vaccination for Children Aged 5 to 11 Years - Paul-Ehrlich-Institut
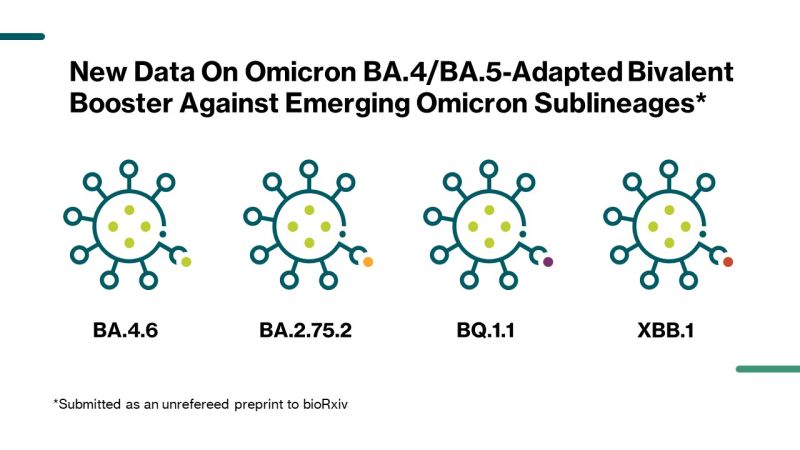
BioNTech SE on LinkedIn: Pfizer and BioNTech Report New Data on Omicron BA .4/BA.5-Adapted Bivalent…
Covid: Aifa approva il vaccino bivalente Comirnaty sviluppato contro Omicron 4-5 | Sanità24 - Il Sole 24 Ore

ATAGI recommendations on use of the Pfizer bivalent (Original/Omicron BA.1) COVID-19 vaccine | Australian Government Department of Health and Aged Care
CDC recommends new COVID booster for all Americans over 6 months amid rising cases, hospitalizations - ABC News